After nearly 10-months of the pandemic jeopardizing our lives, we have some positive COVID-19 vaccine updates. Finally, a few pharma and biotech companies have their vaccine trial reports ready, and luckily, some of the early testers have optimistic results to share. As per the latest vaccination updates, two companies reported 94- and 95-percent efficacy in their final trial.
With a safe and effective COVID-19 vaccine, the pandemic can finally be controlled with normalized life and societal functions.
An effective vaccine is undoubtedly a critical strategy to reduce COVID-19-related illnesses, hospitalizations, and deaths worldwide.
COVID-19 Vaccine Goals:
In the U.S.A, the government aims to have enough vaccines ready for all people who wish to be vaccinated by early or mid-next year. Based on the COVID-19 Vaccination Program’s newest distribution plan, there may be a limited supply of COVID-19 vaccine at the initial stage. As per the plan, only healthcare workers and high-risk populations will receive the vaccination in the first phase. Several pharmaceutical companies are already in their third phase of a vaccine trial, and only two companies have finished their final stage of vaccine trials. While there is not yet an authorized or approved vaccine in the U.S.A., both Pfizer and Moderna are aiming to get emergency approval from the F.D.A. to administer vaccines to prevent the coronavirus disease from spreading further.
“The challenge is, the end isn’t coming soon. But it’s coming, and what we need to do is try to have as few [COVID-19] cases as possible between now and the time a vaccine arrives.“
News:CBC
C.D.C.’s Role:
Although C.D.C. is not actively involved in the COVID-19 vaccine development, it has been working closely with the health departments and partners to develop an effective vaccine distribution plan. C.D.C. is collaborating with local governments and partners at all levels, including many healthcare associations, and creating a flexible COVID-19 vaccination program to accommodate all possible scenarios during the vaccine development and distribution.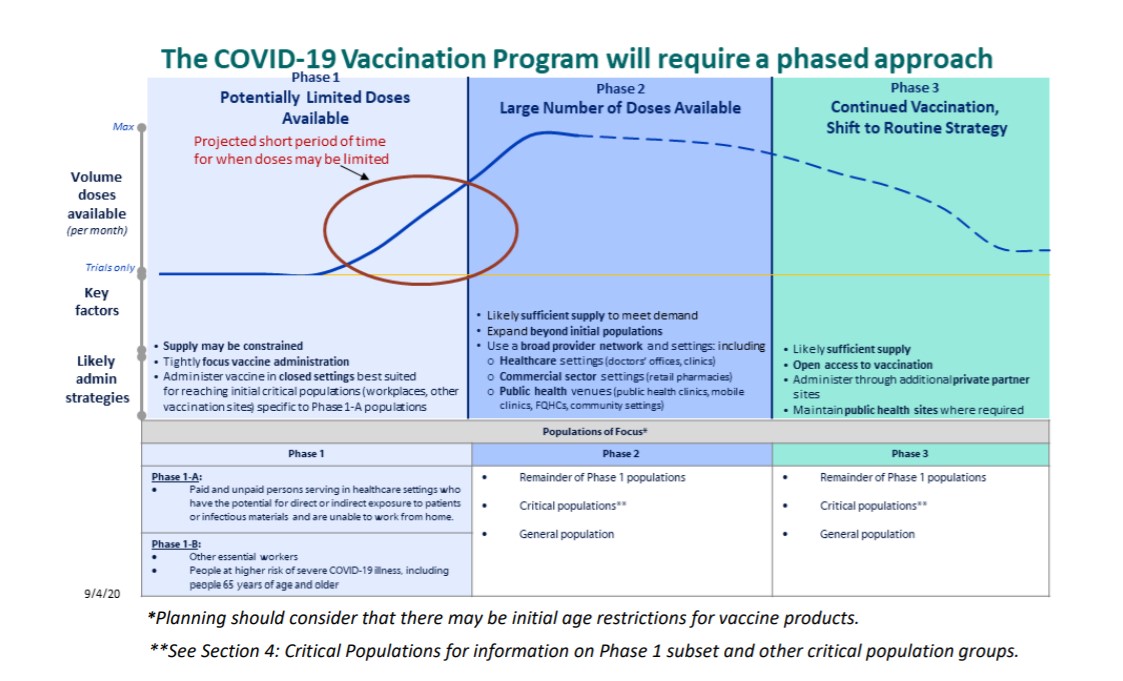
Source: CDC.Gov
Why Should You Take COVID-19 vaccination?
A coronavirus vaccine will help keep you from getting COVID-19 and help you build immunity against the virus. All the COVID-19 vaccines are being carefully evaluated through several clinical trials before getting authorized or approved for public administering.
Based on several studies, the benefits of taking a COVID-19 vaccine are:
- Building immunity against coronavirus
- May help keep you from getting seriously ill with COVID-19
- Helping those, particularly at increased risk for severe illness from the virus
- May help stop the pandemic and return to normal life
However, experts will continue to study the efficacy and effect of the COVID-19 vaccination on the severity of illness and its ability to develop immunity.
COVID-19 Vaccine Updates:
Two American candidates are ahead of all in the COVID-19 vaccine development race, with over 90% accuracy and efficacy in their third phase trial. Using a similar mRNA platform and vaccine development technology, both Pfizer and Moderna are all set to launch the first-ever COVID-19 vaccines in the U.S.A. market.
While the government and F.D.A. are still in the process of validating and approving the vaccines, the study numbers prove that we are heading in the right direction.
Last week, Pfizer and BioNTech released their first set of vaccine third-phase trial data, touting a 90% efficacy rating. Their final numbers are out this week, which blew past their last data with a new 95% rating.
Similarly, Moderna developed the coronavirus vaccine using the mRNA platform, which broke the record with a 94.5% efficacy rating, much higher than the 60% bar set by the Food and Drug Administration.
Image Source: Washington Post
Both vaccine trials surpassed the minimum number of infections set by the F.D.A. in order to conclude the trial. While the Pfizer vaccine requires an arduous ultra-cold temperature for its safe storage, Moderna, on the other hand, doesn’t have any such strict storage and handling needs.
Company History:
Pfizer:
Pfizer, a pharmaceutical company with a massive global presence, is one of the world’s leading coronavirus vaccine developers. With years of experience in vaccine creation and infrastructure to mass-produce, Pfizer can produce a large number of doses in a short period. Tanya Alcorn, Pfizer’s vice president of distribution, recently shared their plans to move forward with ultra-cold freezer purchase to ramp up the distributions once the vaccine is ready. She also shared that Pfizer is trying to create a powder version of the vaccine to eliminate the ultra-cold storage burden.
Moderna:
Moderna’s approach is very different. From a research-based biotechnology company to vaccine development, Moderna’s every movement has an mRNA-based theory. The company’s research and product-line are on the mRNA platform. Their newest endeavor to create a COVID-19 vaccine is also based on the mRNA platform. The result, with an efficacy of 94.5% in their 3rd phase trial, is certainly praiseworthy. The company has recently mentioned that their vaccine has a longer shelf life and doesn’t require any ultra-cold refrigeration for storage.
How Does Moderna’s Coronavirus Vaccine Candidate Compare To Pfizer’s?
While companies are still gathering enough clinical trial data to request for an emergency use authorization from the F.D.A., here are some comparison data based on the earlier reports.
| COMPANY NAME | Moderna | Pfizer |
| Vaccine Efficacy Rating | 94.5% | 90%, 95% |
| Platform | mRNA | mRNA |
| Storage Temperature | Refrigeration & Room Temp | Ultra-cold Refrigeration |
| Trial Conclusion | Effective | Effective |
| Rollout Date | TBD | TBD |
While both companies are using the messenger R.N.A. (mRNA) platform, Moderna’s vaccine is more user-friendly because of its flexible refrigerator storage for up to 30-days.
Availability:
As of now, both vaccines require F.D.A.’s emergency usage approval before distributing them for general administration. After the approval, both companies will have limited, rationed supplies by the end of this year.
Coronavirus Vaccine Rollout Plans:
The strategic plan for the coronavirus vaccine rollout includes a phased approach.
Phase-1:
In the first phase, limited doses of the vaccine will likely be available for the critical populations and essential workers, including
- Healthcare personnel
- Workers in other essential industries
- Those at high risk for severe COVID-19 illness due to underlying health conditions
- Population aged 65 years and older
Phase-2:
With a larger number of doses available, the second phase anticipates the vaccine distribution among a larger population, focusing on critical and specific people.
Phase-3:
The last phase, or phase 3, will likely have enough vaccination supply for wide distribution. This phase will most likely have the vaccine administration integrated into routine vaccination programs for broad access.
Our Responsibility:
Although a vaccine may help stop the spread of the disease, we all should follow social distance guidelines and mask-wearing as responsible citizens. Wearing masks, avoiding small or large gatherings, and social distancing minimizes your chance of being exposed to this virus and stopping the spread. The combined effort of getting vaccinated and following CDC’s recommendations will help all of us to effectively fight COVID-19 and stopping a pandemic.
Axiom Medical manages COVID-19 employee screening and testing at the workplace. Our onsite mobile lab and flu clinic also offer employee vaccination programs to help you manage workplace contagious disease outbreak.
Have Questions? Contact Us Today!
Contact Us
Click Below For Our FREE COVID Vaccine Guide!

With a career focused on digital marketing, Chitra is a specialized SEO-Content marketer. After moving from biotechnology to business operations and marketing, Chitra started her digital marketing career as a freelance content developer and technical writer. With Axiom, as a content marketing & SEO specialist, she is passionate about creating informative marketing copies for optimum search engine performance.
Find out more about our Tempo Live Behavioral Health and Injury Case Management services.











