Following the Food and Drug Administration’s emergency use authorization, two vaccines are now available for Americans. In this fight against the COVID-19 pandemic, Pfizer and Moderna vaccines are the first contenders in the race. With so many other vaccines in the market, it can be confusing to have two different vaccines and possibly, having more to come in the United States to join the race to get people immunized against the same virus. Here is what you need to know about how the Pfizer and Moderna vaccines compare.
Race to Emergency Use Authorization (EUA):
While the pharmaceutical company Pfizer was the first to reach the finish point of vaccine development, the biotechnology company Moderna was the second company to get the EUA from the United States FDA. Now Moderna’s COVID-19 vaccine has joined Pfizer’s in being administered to healthcare workers and residents in long-term care facilities in the United States.
Target population:
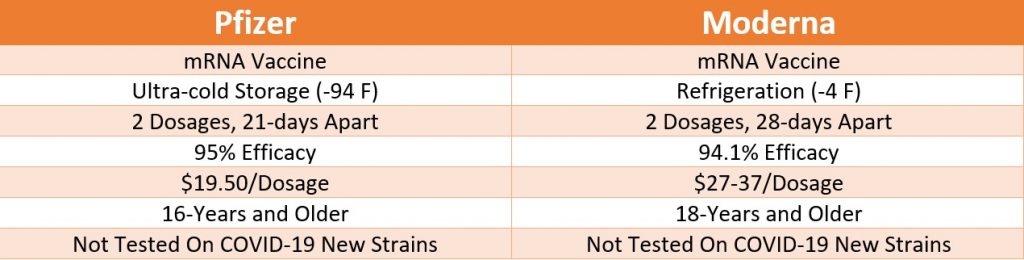
The Pfizer emergency use authorization is for anyone of or above 16 years. In contrast, Moderna’s authorized COVID-19 vaccine is for people 18 and older. Both companies have recently begun testing their vaccine on participants of 12- to 17-year-olds.
Vaccine efficacy:
During the vaccines’ phase-3 clinical trials, both have shown astonishing and nearly equivalent results with close to 95% of efficacy. However, it is still unknown how long each vaccine will provide immunity against the virus. Since both the vaccines are new, there might be a possibility of offering at least one booster dose after the initial vaccination’s first two dosages. Indeed, it remains unclear how both vaccines will stack up over the long term.
Pfizer vs. Moderna COVID-19 Vaccines – Similarities:
m-RNA Based Vaccine:
Most human vaccines are attenuated virus or bacterial based immune-boosting options. However, the COVID-19 vaccines by Pfizer and Moderns work using messenger RNA (mRNA). Unlike traditional attenuated microbial injections, these mRNA vaccines work by encoding a fragment or portion of the SARS-CoV-2 virus’ spike protein into human cells. The fragment stimulates antibody production, which eventually provides an immune response against the virus.
Mechanism:
According to the Centers for Disease Control and Prevention (CDC), once the vaccine is administered, the mRNA will bind to the immune cells, and the human cells use them to make the corresponding protein piece. The encoded proteins, then, spark an immune response that promotes immune response against the COVID-19 virus. Once your body creates the expected immune response, your body eliminates the protein and mRNA while the produced antibodies provide you protection.
Immune Response:
While both vaccines use a similar mRNA platform and immune response mechanism, both vaccines have different immune response levels. Unlike the flu vaccine, no actual virus is used in this case. During its last stage of clinical trial, the Moderna vaccine was 94.1% effective at preventing symptomatic Covid-19. The Pfizer vaccine has a slightly higher efficacy rate measured during the last clinical trial stage.
Both vaccines appeared to be equally effective across different age and ethnic groups and races. If not complete elimination, both vaccines seemed to reduce the risk of severe COVID-19 disease.
Side Effects:
Both Pfizer and Moderna vaccinations have similar side effects. When administered in the deltoid muscle, users have reported the following side effects, including:
- Pain at the administration site
- Tiredness, headache
- Muscle pain, chills
- Joint pain, fever
- Swelling at the injection site and redness
- Nausea and vomiting
Here is more information on CDC’s COVID-19 vaccine recommendation.
While severe reactions were not reported in clinical trials, it is essential to note that the FDA does not currently advise individuals with life-threatening allergies to receive the vaccination at this time.
Pfizer vs. Moderna COVID-19 vaccines – Differences:
The Durability of Protection:
It’s undoubtedly going to take some time to figure out the durability of both the vaccines’ protection. Vaccine developers have plans to perform periodic tests by drawing blood from some volunteers to see their antibody levels. However, experts have confirmed that a decline in antibody levels doesn’t necessarily equate to reducing the immune response against the virus.
This process will also involve watching reports that the immunized population is starting to contract COVID-19 disease in larger numbers. In such a situation, there might be a recommended booster shot at some interval.
While both vaccines have nearly similar immune responses, the Pfizer vaccine showed the efficacy of 95%, and the Moderna showed the efficacy of 94%. Both vaccines were measured based on providing immune response and shipping symptomatic COVID infection.
Storage:
Even though both vaccines use a similar mRNA platform, only Pfizer has a more stringent storage requirement. Currently, the storage requirement is one of the most significant factors for Pfizer’s vaccine distribution. This is also one of the most significant differences noted between the Pfizer-BioNTech and Moderna vaccine.
Pfizer vaccine has an ultra-cold storage requirement, which technically makes it challenging to store and distribute appropriately. A freezer unit with -94 Fahrenheit is required to ship and store the Pfizer vaccine. However, the company has bought several ultra-cold storage freezers to ease the vaccine distribution burden. While storage and distribution may not be an issue for hospitals and healthcare organizations, small distribution chains and public health clinics may find this to be an ongoing concern with regards to vaccine storage and distribution. After the thawing, the Pfizer vaccine must be administered within five days.
In contrast to Pfizer, Moderna’s vaccine doesn’t require ultra-cold temperature for storage and distribution. The Moderna’s vaccine must be shipped at -4 Fahrenheit, the temperature in a regular refrigerator. Moderna’s vaccine stays stable in the fridge for up to 30-days and up to 12-hours at room temperature after thawing.
Allergic Reaction:
Studies on the Moderna vaccine also claim that the vaccine may interfere and cause allergic reactions in people with facial fillers.
COVID-19 New Strains:
Both vaccines are now tested against the newly discovered, highly contagious COVID-19 strains.
Which COVID-19 vaccine is the best?
Both Moderna and Pfizer are equally efficient and provide an immune response against the Coronavirus strain that causes COVID-19. There is no “best” vaccine option to stop the spread of the disease. While vaccines can help build immunity, there is not a silver bullet to stop the pandemic. In addition to the vaccinations, we should wear masks, follow hand hygiene, and social distancing to help our community fight the disease.
Axiom Medical is a long-time occupational health service provider and specializes in workplace COVID-19 management. Call us today and let us help you.
Interested to learn more?
Contact Us
Get Our FREE COVID Vaccine Guide!

With a career focused on digital marketing, Chitra is a specialized SEO-Content marketer. After moving from biotechnology to business operations and marketing, Chitra started her digital marketing career as a freelance content developer and technical writer. With Axiom, as a content marketing & SEO specialist, she is passionate about creating informative marketing copies for optimum search engine performance.
Find out more about our Tempo Live Behavioral Health and Injury Case Management services.