 Although many countries had the ambitious goal to produce the COVID-19 vaccine by the year-end, the timeline expectations are running up against the reality. In the blog post, we will walk you through the latest developments in COVID-19 vaccines and therapeutics.
Although many countries had the ambitious goal to produce the COVID-19 vaccine by the year-end, the timeline expectations are running up against the reality. In the blog post, we will walk you through the latest developments in COVID-19 vaccines and therapeutics.
A Message from Our Chief Medical Officer, Scott Cherry
Newest CDC Guidelines
- For individuals that are exposed to COVID-19 via close contact
(but do not become symptomatic or test positive), the social
isolation guidance has been to isolate for 14 days. - New guidance from the CDC on 12/2/20 is now recommending a
shorter isolation period of 10 days instead of 14 days. - Another component to the updated guidance is that a negative
COVID-19 test would allow the isolation period to be further
shortened to 7 days. CDC estimated the residual risk using this
strategy would be 5% on average, with an upper limit to 12%.
In summary, the new guidance is a step to broadly push the critical infrastructure exception (where these employees who are exposed, but remain asymptomatic/test negative, can remain at work with a layered approach to protection).
Current Coronavirus Status In The USA:
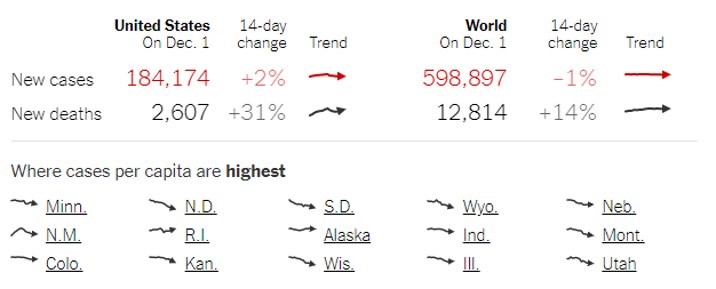
With more than 200,000 cases a day and a soaring number of fatalities, the USA’s current coronavirus status looks grim and horrifying. The Centers for Disease Control (CDC) has repeatedly warned that this winter may be the dreadful one and one of the most challenging times in US public health history. With the alarming number of hospitalizations and cases per day, our country has surpassed its previous metrics in a single day.
In an effort to minimize the risk of coronavirus spreading, several states have imposed mandatory quarantine and curfew during this holiday time. CDC also reduced the mandatory quarantine period from 14-days to 7-days with diagnostic tests and symptoms monitoring.
With more than 11 million confirmed cases, the US has the highest number of infections globally, and the spread of the virus shows no sign of slowing down. The above chart indicates that the current wave is growing at a faster rate than the previous one. In such a dire situation, the only thing we can do is practice hand hygiene, limit our social activities, and maintain social distance whenever in public.
COVID-19 Therapeutics:
The Food and Drug Administration (FDA) has approved Remedesivir, an antiviral drug, only for hospitalized COVID-19 patients. Bamalanivimab and Caririvimab with Imdevimab are two antibody therapies (monoclonal antibodies) used for mild to moderated COVID-19 patients, including adults and pediatric patients. People with COVID-19 showing severe symptoms should receive supportive care to help relieve symptoms.
Besides, self-care becomes very important for all patients with COVID-19 showing mild to moderate symptoms.
- Remdesivir – Antiviral for Hospitalized COVID-19 Patients
- Bamlanivimab – Antibody Therapy for Mild-to-Moderate COVID-19 in
Adult and Pediatric Patients - Casirivimab + Imdevimab – Mild-to-Moderate COVID-19 in Adults and
Pediatric Patients
Self-care Suggestions:
For those with possible or confirmed COVID-19:
- Stay home and quarantined until you test negative.
- Carefully monitor your symptoms. Seek help from your primary healthcare provider if your symptoms get worse.
- Drink plenty of water and take a rest. For fever and body pain, take over-the-counter medicines, such as acetaminophen.
- If you have an underlying health condition, keep a close watch on your symptoms.
- If you are not staying alone, wear a mask when you must be around others.
COVID-19 Vaccine Timeline/Expectations:
While several companies completed their last phase of the COVID-19 vaccine in November, there is still time for the vaccine to be available to the public. Without a doubt, we all probably need to reset our expectations about how quickly we can be vaccinated.
Moderna and Pfizer are the two candidates in the USA well ahead of others in the COVID-19 vaccine development race, with over 90% accuracy and efficacy in their third phase trial. Using a similar mRNA platform and vaccine development technology, both Pfizer and Moderna are all set to launch the first-ever COVID-19 vaccines in the USA market.
In November, Pfizer and BioNTech released their first set of vaccine third-phase trial data, touting a 94 and 95% efficacy rating. While the Pfizer vaccine requires an arduous ultra-cold temperature for its safe storage, Moderna, on the other hand, doesn’t have any such strict storage and handling needs.
At a recent press conference, Pfizer’s spokesperson shared that their company aims to vaccinate most Americans by the March and April timeframe. CDC is working on expanding safety surveillance through new systems and additional information sources and scaling up existing safety monitoring systems.
Will the COVID-19 Vaccine Be Safe?
Vaccines go through several trials before being available for public usage. The efficacy data of both the USA vaccines seem to be very safe. Even after a vaccine is authorized or approved for use by the FDA, vaccine safety monitoring systems will be used to watch for possible side effects and adverse reactions after the vaccine injections. Considering the efficacy rating after the third trial, which included many older persons, the vaccine safety may not be a primary concern. Based on the future vaccine safety monitoring, experts may decide whether changes are needed in the USA to ensure that the benefits continue to outweigh the risks for those taking the vaccines.
Safety & Security – Our Responsibility:
Although the new COVID-19 vaccine may help stop the disease spread, it may not eliminate it. As responsible citizens, we all should follow social distance guidelines, hand hygiene, and social distancing. Wearing masks, avoiding small or large gatherings, and social distancing minimizes your chance of being exposed to this virus and stopping the spread. 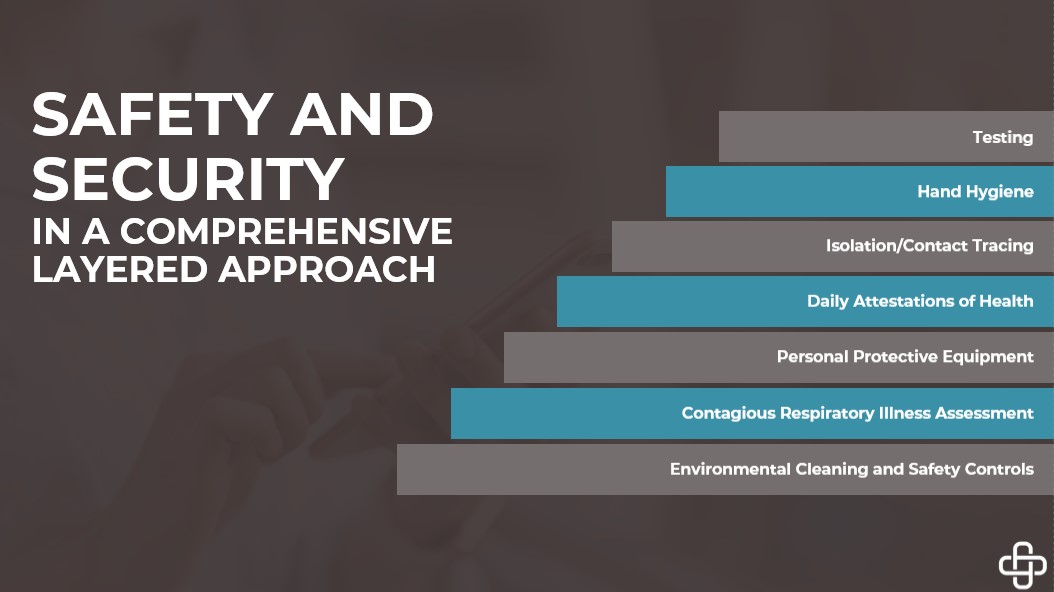
The combined effort to get vaccinated and follow CDC’s recommendations will help us fight COVID-19 and stop a pandemic effectively.
Listen to our expert panel discussing this topic. Click below to watch the on-demand video.











